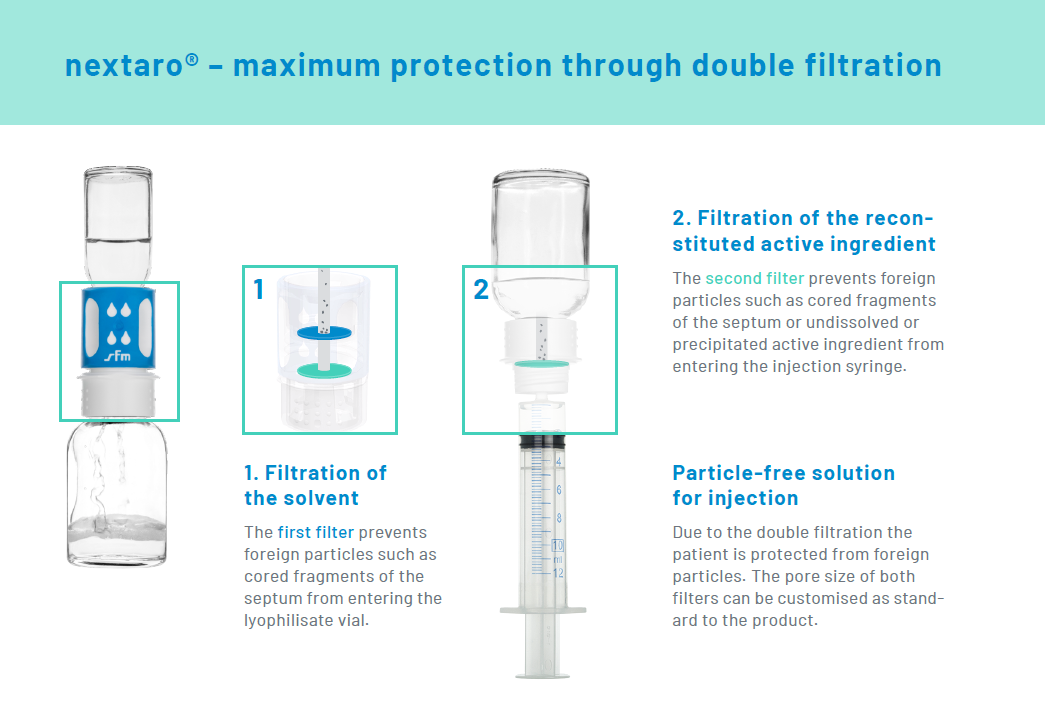
With the reconstitution of lyophilized drugs it is possible that when the septum of the vial is punctured, minute particles are punched out.1 These could enter the body when the drug is administered. The premium adapter nextaro® minimizes this risk with its carefully designed filter system.
Foreign particles due to reconstitution
The injection of lyophilized drugs is part of everyday life for medical facilities and chronically ill patients. However, with the reconstitution of active ingredients foreign particles may be generated which could lead to health implications that cannot as yet be estimated. Minute particles may form inter alia due to
- Coring/abrasion of the septum on drawing up of the solvent and on transfer into the active ingredient vial;
- Incomplete dissolution of the active ingredient;
- Precipitates. They form for instance if a liquid is used with a drug that has already been dissolved for the reconstitution of an additional substrate and the active ingredients are not compatible. However, variations in temperature, shearing forces or changes in pH can also lead to the precipitation of the active ingredient.
Despite filtration there is a risk
Reconstituted active ingredient solutions should be filtered prior to injection in order to prevent an administration of foreign particles.2 In many systems no filter or only one filter is used for this: one side filters the solvent before it meets the active ingredient. The other side filters the reconstituted active ingredient. However, this method carries the risk that particles, which were initially captured from the solvent, enter the syringe when it is drawn up - and thus enter the body as well.
Particle-free injection solution - with nextaro®
The premium adapter nextaro® with its carefully designed filter system prevents this risk. It comprises two different filters:
- A first filter removes contaminants from the solvent.
- A second filter prevents the drawing up of particles into the injection syringe.
- The pore size of both filters can be customised as standard to the active ingredient.
With nextaro® an easy to handle and customisable premium adapter is thus available, which with its intelligent design minimizes the risk of particulate contamination. Therefore nextaro® offers maximum protection for end users and patients.
Literature:
- Besheer A, Mahler H-C, Matter-Schwald A et al. Evaluation of Different Quality-Relevant Aspects of Closed System Transfer Devices (CSTDs). Pharmaceutical Research 2020; 37: 81. https://doi.org/10.1007/s11095-020-02784-1
- CIRS-AINS Spezial: Partikelkontamination nach dem Aufziehen von Arzneimitteln - Ein relevantes, aber lösbares Problem. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2013; 107: 352-355. https://doi.org/10.1016/j.zefq.2013.05.012