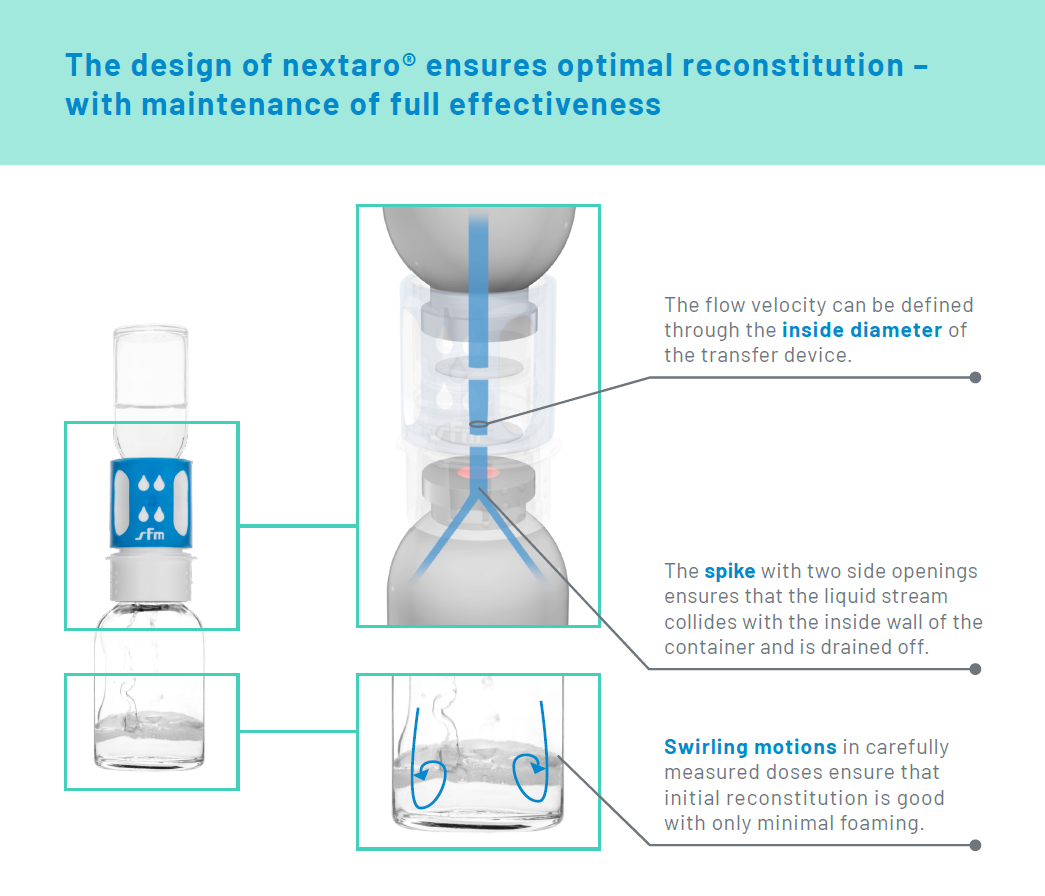
Foam formation is one of the greatest challenges faced in the reconstitution of pharmaceutical lyophilisates - and should be averted. This is because foam can limit the effectiveness of the product and delay administration. The premium transfer devicenextaro® prevents "foaming" with its unique design.
Anyone working with lyophilisates is familiar with the problem: a clear liquid, an expensive lyophilisate (often protein-based), the moment when the two meet – and yet again foam. Foam formation with lyophilisates is undesirable because:
- The active ingredient molecules can oxidise and denaturise on the surface of the foam. In this way the solution contains less active substance with the result that the effectiveness of the product may diminish. The therapeutically active proteins that are frequently used are particularly affected by this.
- Until the foam recedes, valuable time is lost. With acute and urgent applications a further active ingredient reconstitution may then have to be carried out, the "failed" reconstitution would have to be discarded. Thus, valuable substances would end up in the waste needlessly and push up costs.
Reconstitution - from a physical point of view
The lyophilisate starts to dissolve as soon as it meets with a liquid. In the process an interface layer forms, which consists of a saturated solution of the powder in the solvent1. The active ingredient molecules have to penetrate this interface layer in order to continue and complete the dissolution process – with no swirling motions in the liquid this takes place purely through diffusion, which can considerably slow the process1. Swirling motions in the liquid help to rapidly break up the interface layers that have formed and quickly dissolve the lyophilisate. However, depending on the design of the adapter, when swirling motions are generated, unwanted foaming may occur.
Prevent foaming – with the right equipment
With the premium transfer device nextaro® the necessary swirling motions are generated – and at the same time foaming is averted. The following mechanisms prevent foam formation:
- Regulation of flow velocity
The flow velocity of the liquid is set by the inside diameter of the connector and can be customised as standard. - Draining off of liquid to the sides
The spike, from which the liquid flows into the active ingredient vial, has two side openings. They ensure that the liquid stream collides with the inside wall of the container and is drained off.
Thus, when liquid and active ingredient meet at the bottom of the vial, swirling motions are generated in carefully measured doses – and initial reconstitution is good with only minimal foaming. In order to obtain complete dissolution the user also has to gently move the vial from side to side as appropriate.
In which areas of medicine can nextaro® be used?
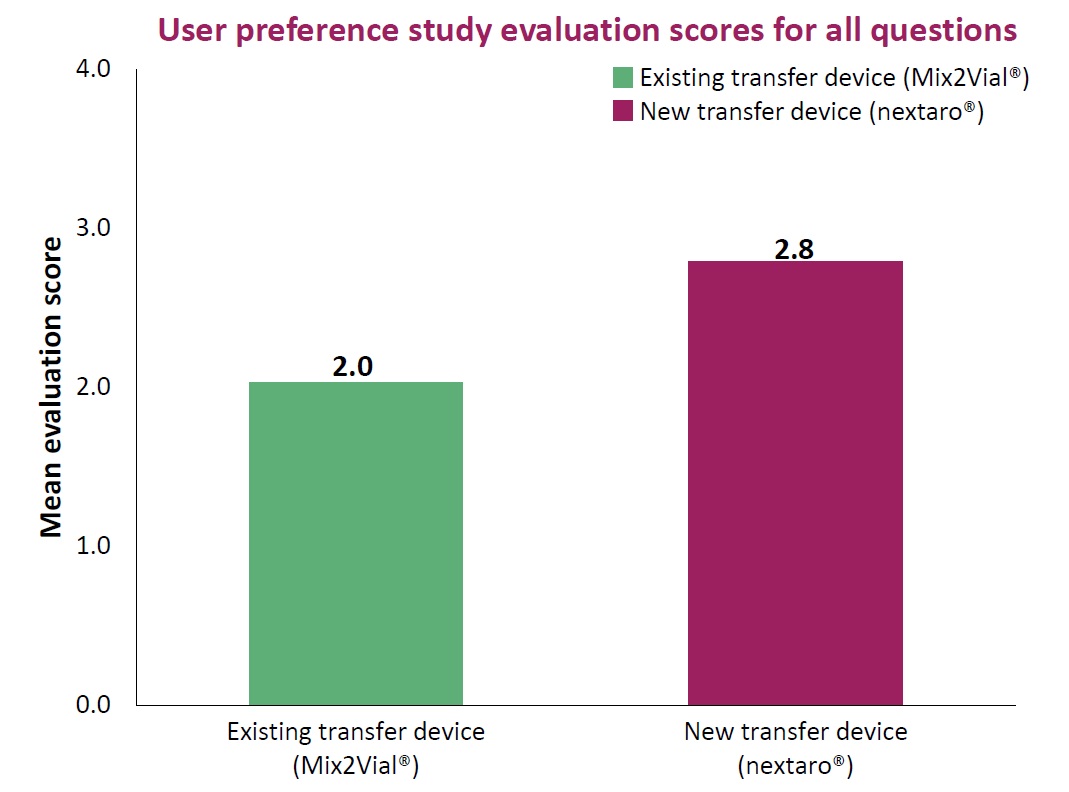
At present there are many pharmaceutical agents available as lyophilisate. Current areas of application include the fields of multiple sclerosis, haemophilia and stroke. In addition, agents in the form of lyophilisates are increasingly being developed for future indications. Whether administered by the physician, nursing staff or carer or used by the patient himself: with nextaro® an easy to handle, robust and customisable premium device is available, with which the reconstitution of lyophilized substances can be achieved quickly and simply. As a result nextaro® is setting new standards.
Literature:
- Harvey C. Reconstitution devices: Mixing, power, packaging & regulation. ONdrugDelivery 2018; 2018: 32-35