The vacuum in an active ingredient vial plays an important role when reconstituting lyophilisates: In non-ventilated transfer systems, the negative pressure ensures that the solvent is completely transferred. If the vacuum is lost due to a faulty connection, the reconstitution will fail partly or completely. The nextaro® premium adapter is designed to prevent this from happening.
Negative pressure is created in the vial through the freeze-drying process of the active ingredient. This negative pressure is essential for the successful reconstitution of non-ventilated transfer systems; it ensures that the solvent is drawn in through the transfer system.
With conventional transfer systems that do not feature an integrated guiding system for connecting the vial, the septum of the vial that contains the active ingredient can be pierced at an angle. This means that air can be drawn in, which destroys the vacuum and makes complete reconstitution impossible. If this happens, the vial and its valuable active ingredients must be discarded. This wastes time, which can be critical in an emergency. The considerable additional costs of expensive active ingredients must also be taken into account.
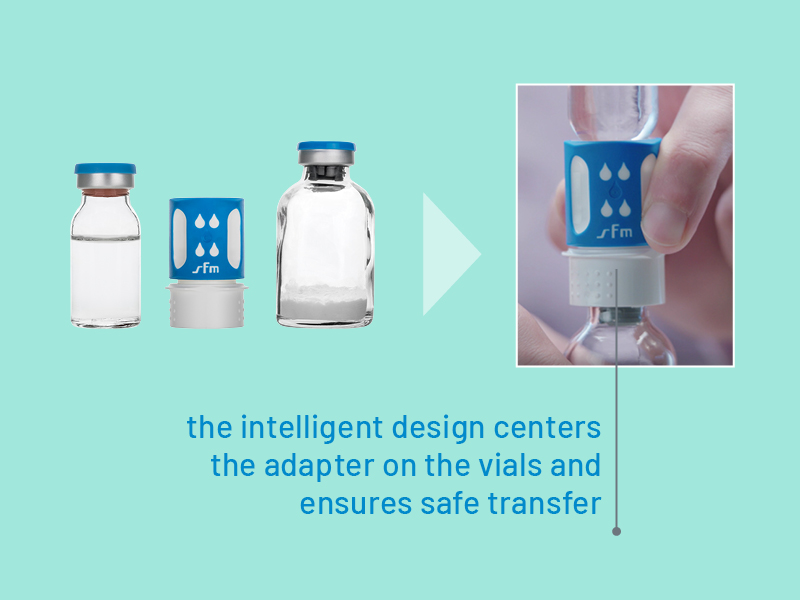
The correct line-up – with nextaro®
The design of the nextaro® premium adapter prevents unintentional vacuum loss. The correct order for connecting the transfer system to the two vials is clearly identifiable. Its guiding shape means that the transfer system is automatically centered so that the vial cannot be pierced at an angle. And even if the nextaro® is initially tilted at the wrong angle, its stable and innovative structure ensures that the transfer system is guided into the right position, as confirmed by laboratory tests.
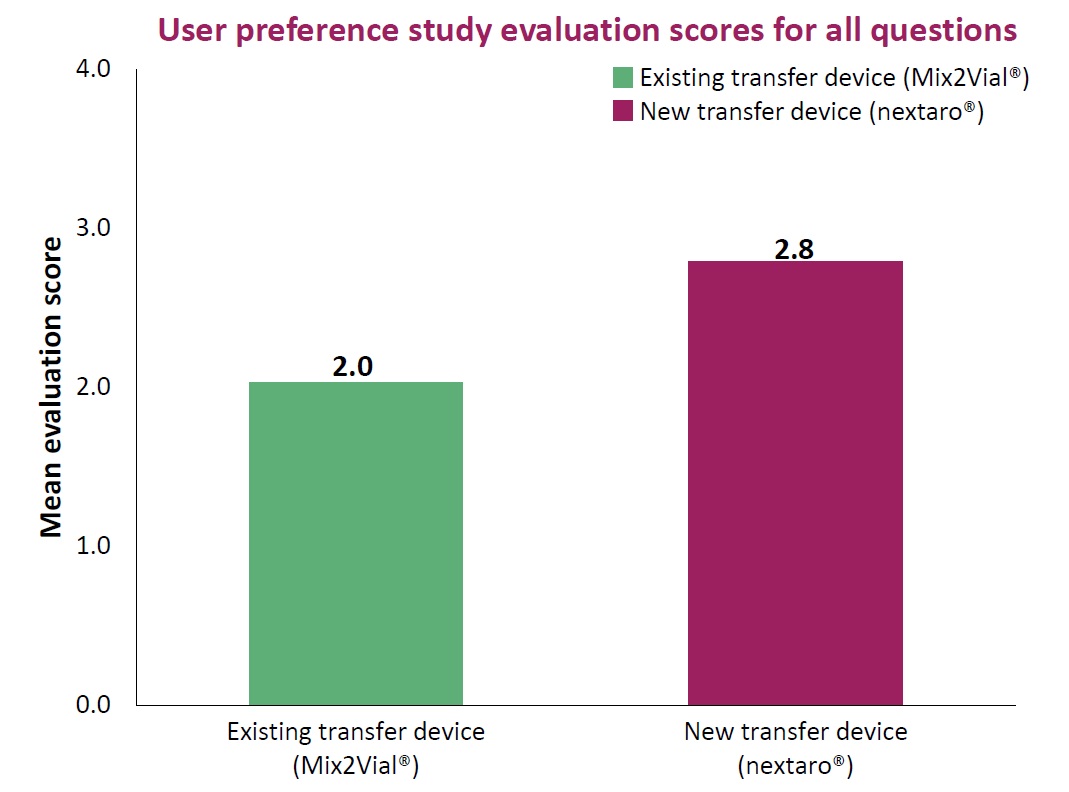
The premium adapter helps to prevent application errors – and therefore minimizes potential additional costs.